Thursday, January 6, 2011
Tuesday, January 4, 2011
Factors that Affct the Rate of Chemical Reactions (The Sad Attempt at a Harried Continuation)
As anyone ruminating attentively on the topic might come to be enlightened to at some random point not necessarily within utterly academic hours, it's useful to be able to predict whether an action will affect the rate at which a chemical reaction proceeds. However, knowing that much would be undermined by oblivion as to why the reaction rate is thus affected. Though there are several factors that can influence the rate of a chemical reaction, generally, a factor that increases the number of collisions between particles increases the reaction rate.
A higher concentration of reactants leads to more effective collisions per unit time, which leads to an increasing reaction rate (except for zero order reactions). . As the concentration of the reactants increases, the reaction rate increases:
 =>
=>
 =>
=>

Catalysts, substances that alters the rate of a chemical reaction without being used up or permanently changed chemically, lower the activation energy of a chemical reaction and increase the rate of a chemical reaction without being consumed in the process. Catalysts work by increasing the frequency of collisions between reactants, altering the orientation of reactants so that more collisions are effective, reducing intramolecular bonding within reactant molecules, or donating electron density to the reactants. The presence of a catalyst helps a reaction to proceed more quickly to equilibrium. Aside from catalysts, other chemical species can affect a reaction. The quantity of hydrogen ions (the pH of aqueous solutions) can alter a reaction rate. Other chemical species may compete for a reactant or alter orientation, bonding, electron density, etc., thereby decreasing the rate of a reaction.
 The above graph demonstrates what a catalyst does to the reaction profile.
The above graph demonstrates what a catalyst does to the reaction profile.
Concentration of Reactants
A higher concentration of reactants leads to more effective collisions per unit time, which leads to an increasing reaction rate (except for zero order reactions). . As the concentration of the reactants increases, the reaction rate increases:
- WHY?
- As the Collision Theory dictates, the reaction rate is directly proportional to the number of effective collisions per second between the reactant molecules.
- Effective Collisions - the fraction of total collisions that actually result in the formation of the product(s). To achieve this, the colliding particles must be in the correct orientation. The Activation Energy (Ea), which is the minimum amount of energy required to activate atoms or molecules with sufficient Kinetic energy to move around more so as to ensure collisions that will successfully result in a chemical reaction, is also prerequisite.
- If the concentration of the reactants increases (i.e. particles per given volume) the greater the number of total collisions due to the resulting decrease in spaces between the reacting particles. With less distance to travel inside the vessel, the more frequent collisions become.
- The greater the frequency of total collisions, the greater the frequency of effective collisions.
- As the frequency of effective collisions increases, so does the reaction rate.
- As the Collision Theory dictates, the reaction rate is directly proportional to the number of effective collisions per second between the reactant molecules.
If reaction is between a substance in solution and a solid, you just vary the concentration of the solution. The experiment is straightforward. If the reaction is between two solutions, you have a slight problem. Do you vary the concentration of one of the reactants or vary the concentration of both? You might find that the rate of reaction is limited by the concentration of the weaker solution, and increasing the concentration of the other makes no difference. What you need to do is fix the concentration of one of the reactants to excess. Now you can increase the concentration of the other solution to produce an increase in the rate of the reaction.
- e.g. Increasing the concentration of acid molecules increases the frequency or chance at which they hit the surface of marble chips to dissolve them (slower => faster, illustrated below)
 =>
=>
 =>
=>
- Increasing the concentration of reactant A or B will increase the chance or frequency of collision between them and increase the speed of product formation (slower => faster).
 Graph 4.7 shows the rate/speed of reaction is often proportional to the concentration of one particular reactant. This is due to the chance of a fruitful collision forming products being proportional to the concentration. The initial gradient of the product-time graph e.g. for gas in cm3/min (or s for timing the speed/rate) gives an accurate measure of how fast the gaseous product is being formed. The reciprocal of the reaction time, 1/time, can also be used as a measure of the speed of a reaction. The time can e.g. represent how long it takes to make a fixed amount of gas, or the time it takes for so much sulfur to form in the sodium sulfate - hydrochloric acid reaction. The time can be in minutes or seconds, as long as you stick to the same unit for a set of results for a set of experiments varying the concentration or mass of one of the reactants.
Graph 4.7 shows the rate/speed of reaction is often proportional to the concentration of one particular reactant. This is due to the chance of a fruitful collision forming products being proportional to the concentration. The initial gradient of the product-time graph e.g. for gas in cm3/min (or s for timing the speed/rate) gives an accurate measure of how fast the gaseous product is being formed. The reciprocal of the reaction time, 1/time, can also be used as a measure of the speed of a reaction. The time can e.g. represent how long it takes to make a fixed amount of gas, or the time it takes for so much sulfur to form in the sodium sulfate - hydrochloric acid reaction. The time can be in minutes or seconds, as long as you stick to the same unit for a set of results for a set of experiments varying the concentration or mass of one of the reactants. RATE LAW: an expression relating the rate to the concentrations of reactants.
For a general reaction between reactant A and B at a constant temperature the reaction may be represented by the following equation. The small letters a and b represent the coefficients used to balance the reaction.
aA + bB -------> productsAs we saw in the previous lesson, the rate of a chemical reaction generally increases with reactant concentrations. The rate of the equation above can be represented by the following relationship
This rate relationship can be expressed as a rate law. The rate law equation expresses the relationship between the concentration of the reactants and the rate of the equation.Rateproportional to[A] mx[B] n
Also notice in the the presence of the rate constant k. Students often have trouble distinguishing between the rate of a reaction and its rate constant.The rate of a reaction is the total rate of a reaction and is the "rate" in the rate law. The units of rate are always M / s. The rate constant, k, is an experimentally determined proportionality constant that gives some measure of the intrinsic "reactivity" of the reaction. The units on the rate constant depend on the order of the reaction, n, such that k has units M1 - n / s. In that way, the units of the rate constant, k, are chosen to make the units of rate always M / s regardless of order. For example, if the order of the reaction is 3, the rate law could be rate = k [A]2[B]. The units of rate are M / s and the rate constant must, therefore, have units of M-2 / s.
Rate Law Problems
Orders of Reactions; Determining the Rate LawNote the following about the equation above.
Rate constant (k)Exponents m and n1 varies with temperatureconstant with temperature2 constant under constant conditionscan only be determined experimentally
The initial concentrations of H2 and I2 are equal at all times and the initial concentration of product is zero: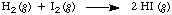
As you can see, the rate of formation of HI is twice the rate of disappearance of H2 or I2 at any given time. Also, note that the rate slows in time due to decreasing concentrations of the reactants. Stated mathematically, the relationship between the formation of products and the disappearance of reactants for this reaction is: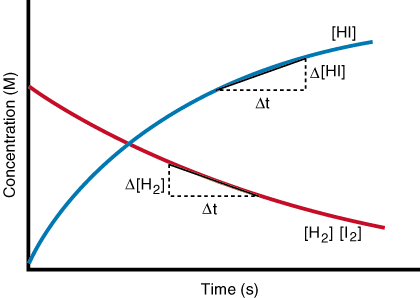
Figure %: Graph of concentration versus time for the reaction of hydrogen and Iodine
In general, for the reaction below: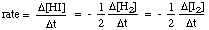
The rate is expressed as follows: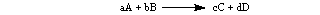
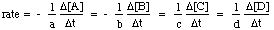
Temperature
Temperature is a measure of the kinetic energy of a system, so higher temperature implies higher average kinetic energy of molecules and more collisions per unit time. A general rule of thumb for most (not all) chemical reactions is that the rate at which the reaction proceeds will approximately double for each 10°C increase in temperature. Once the temperature reaches a certain point, some of the chemical species may be altered (e.g., denaturing of proteins) and the chemical reaction will slow or stop. As the temperature of a system increases, the reaction rate increases. - WHY?
- Temperature (T) - A measure of the average kinetic energy (KEavg) of the particles of a substance.
- Increasing T increases KEavg.
- At higher T, the fraction of molecules with energies greater than the activation energy (Ea) increases.
When gases or liquids are heated the particles gain kinetic energy and move faster (see diagrams below). The increased speed increases the frequency of collision between reactant molecules and the rate increases. BUT this is NOT the main reason for the increased reaction speed, so read on after the pictures!
 =>
=>
Before any change takes place on collision, the colliding molecules must have a minimum kinetic energy called the Activation Energy shown on the energy level diagrams below (sometimes called reaction profile/progress diagrams - shown below). Going up and to the top 'hump' represents bond breaking on reacting particle collision. The purple arrow up represents this minimum energy needed to break bonds to initiate the reaction, that is the activation energy. Going down the other side represents the new bonds formed in the reaction products.
The red arrow down represents the energy released - exothermic reaction. It does not matter whether the reaction is an exothermic or an endothermic in terms of energy change, its the activation energy which is the most important factor in terms of temperature and reaction speed. Now heated molecules have a greater average kinetic energy, and so at higher temperatures, a greater proportion of them have the required activation energy to react.
This means that the increased chance of 'fruitful' higher energy collision greatly increases the speed of the reaction, depending on the fraction of molecules with enough energy to react. For this reason, generally speaking, and in the absence of catalysts or extra energy input, a low activation energy reaction is likely to be fast and a high activation energy reaction much slower, reflecting the trend that the lower the energy barrier to a reaction, the more molecules are likely to have sufficient energy to react on collision. 

- CAN YOU DETERMINE THE VALUE OF THE ACTIVATION ENERGY FOR A GIVEN REACTION? IF SO, WHAT DATA DO WE NEED? AND HOW DO WE DO THE CALCULATION?
- The Arrhenius equation quantitatively describes the relationship between the rate constant k, temperature and the activation energy. The rate constant value increases with increase in temperature and nothing else varies it!
- k = A exp(-Ea/RT)
- k = rate constant (from the rate expression)
- A = a constant for a given reaction, sometimes called the 'frequency' or 'pre-exponential' factor and it seems to be linked to stereochemical factors i.e. the spatial aspects of reactant particle collision.
- Ea = activation energy in Jmol-1
- R = ideal gas constant = 8.314 Jmol-1K-1
- T = temperature in K (Kelvin = oC + 273)
- Rewriting the Arrhenius equation in logarithmic form gives:
- ln(k) = ln(A) - Ea/(RT)
- or log10(k) = log10(A) - Ea/(2.303RT)
- From accurate rate data at different temperatures (e.g. 5 or 10o intervals and a minimum of four results) you can calculate the values of k OR more simply, for a fixed 'recipe', a set of 'rate' results.
- You then plot the value of ln(k or relative rate) versus the reciprocal temperature in Kelvin.

- The negative gradient of the graph is equal to -Ea/R,
- so, Ea = -R x gradient (in J, and /1000 => kJ).
- In terms of y = mx + c, m = gradient = dy/dx = Ea/R, c = a constant = ln(A),
- so, in terms of the Arrhenius equation, the algebra equates to
- ln(k) = - Ea/(RT) + ln(A
 | James Clark Maxwell |  | |
Ludwig Boltzmann |  | ||
| A Maxwell-Boltzmann diagram shows the distribution of molecules at differing kinetic energy levels in a gas sample. At higher temperature, a larger fraction of molecules has kinetic energy equal to or greater than the activation energy (Ea) for the reaction than at lower temperature. In other words, a greater proportion of molecules have enough kinetic energy to participate in the reaction. | |||
|
 Graph 4.3 shows the decrease in reaction time with increase in temperature as the reaction speeds up. The reaction time can represent how long it takes to form a fixed amount of gas in e.g. in the first few minutes of a metal/carbonate - acid reaction, or the time it takes for so much sulphur to form in the sodium thiosulphate - hydrochloric acid reaction. The time can be in minutes or seconds, as long as you stick to the same unit for a set of results e.g. a set of experiments varying the concentration of one of the reactants. Graph 4.3 shows the decrease in reaction time with increase in temperature as the reaction speeds up. The reaction time can represent how long it takes to form a fixed amount of gas in e.g. in the first few minutes of a metal/carbonate - acid reaction, or the time it takes for so much sulphur to form in the sodium thiosulphate - hydrochloric acid reaction. The time can be in minutes or seconds, as long as you stick to the same unit for a set of results e.g. a set of experiments varying the concentration of one of the reactants. |
 Graph 4.4 shows the increase in speed of a reaction with increase in temperature as the particles have more and more kinetic energy. The rate of reaction is proportional to 1/t where t = the reaction time. Graph 4.4 shows the increase in speed of a reaction with increase in temperature as the particles have more and more kinetic energy. The rate of reaction is proportional to 1/t where t = the reaction time. |
| Generally an increase of every 10oK in temperature doubles the rate. As the temperature increases the velocity of molecules also increases which results in the increase in the frequency of collision. The rise in temperature rises the kinetic energy of each molecule. It has been found that by raising the temperature by 10k,the fraction of molecule possessing threshold or activation energy becomes double. As a result the no of effective collision is also double,hence rate is doubled. | |
Presence of Catalysts and Competitors
- Possible ways of lowering the Ea of a reaction:
- Increases the frequency of collisions between the reactant molecules.
- Changes the relative orientation of the reactant molecules.
- Donates electron density to the reactant molecules.
- Reduces the intramolecular bonding within the reactant molecules.
- Provides an alternate pathway or mechanism for the reaction.
| ![[Image]](http://library.thinkquest.org/C006669/media/Chem/img/Graphs/Catalyst.gif) |
- Examples of common catalysts:
- Platinum
- Nickel
- Manganese Dioxide (MnO2)


- Therefore at the same temperature, more reactant molecules have enough kinetic energy to react compared to the uncatalysed situation. The catalyst does NOT increase the energy of the reactant molecules!
Black manganese(IV) oxide (manganese dioxide) catalyses the decomposition of hydrogen peroxide.
hydrogen peroxide ==> water + oxygen
2H2O2(aq) ==> 2H2O(l) + O2(g)
- The manganese is chemically the same at the end of the reaction but it may change a little physically if its a solid.In the hydrogen peroxide solution decomposition by the solid black catalyst manganese dioxide, the solid can be filtered off when reaction stops 'fizzing' i.e. all of the hydrogen peroxide has reacted-decomposed. After washing with water, the catalyst can be collected and added to fresh colourless hydrogen peroxide solution and the oxygen production 'fizzing' is instantaneous! In other words the catalyst hasn't changed chemically and is as effective as it was fresh from the bottle!Note: At the end of the experiment the solution is sometimes stained brown from minute manganese dioxide particles. The reaction is exothermic and the heat has probably caused some disintegration of the catalyst into much finer particles which appear to be (but not) dissolved. In other words the catalyst has changed physically BUT NOT chemically.
- Different reactions need different catalysts and they are extremely important in industry: examples ..
- nickel catalyses the hydrogenation of unsaturated fats to margarine
- iron catalyses the combination of unreactive nitrogen and hydrogen to form ammonia
- enzymes in yeast convert sugar into alcohol
- zeolites catalyse the cracking of big hydrocarbon molecules into smaller ones
- most polymer making reactions require a catalyst surface or additive in contact with or mixed with the monomer molecules.
- Enzymes are biochemical catalysts .They have the advantage of bringing about reactions at normal temperatures and pressures which would otherwise need more expensive and energy-demanding equipment.
Surface area of reactants/ solid reactant particle size
In heterogeneous reactions, the rate of reaction depends upon the surface area of solid reactant. Greater the surface area, higher is the rate of reaction. For example finely divided calcium carbonate (marble) reacts more quickly with hydrochloric acid than calcium carbonate chips. It is due to the fact that powered calcium carbonate offers larger surface area to the reacting acid. In other words, by increasing the surface area of reactant, rate of reaction increases due to greater contact between individual particles and also due to the fact that the surface molecules reacts more quickly.
| ||
If one of the reactants is a solid, the surface area of the solid will affect how fast the reaction goes. This is because the two types of molecule can only bump into each other at the liquid solid interface, i.e. on the surface of the solid. So the larger the surface area of the solid, the faster the reaction will be.Smaller particles have a bigger surface area than larger particle for the same mass of solid. There is a simple way to visualize this. Take a loaf of bread and cut it into slices. Each time you cut a new slice, you get an extra surface onto which you can spread butter and jam. The thinner you cut the slices, the more slices you get and so the more butter and jam you can put on them. This is "Bread and Butter Theory". You should have come across the idea in your biology lessons. By chewing your food you increase the surface area so that digestion can go faster.
Pressure on the reaction between two gasses
- If one or more of the reactants is a gas then increasing pressure will effectively increase the concentration of the reactant molecules and speed up the reaction.
- The particles are, on average, closer together and collisions between the particles will occur more frequently.
- The A and B particle diagrams above could represent lower/higher pressure, resulting in lesser or greater concentration and so slower or faster reaction all because of the increased chance of a 'fruitful' collision.
- Solid reactants and solutions are NOT affected by change in pressure, there concentration is unchanged.
Here are some links to helpful additions for clarifications:
- Chemistry: Rate Laws and Kinetics (First or Second Order)
Quiz on the Topic1 Quiz on the Topic 2 - Quiz on the Topic 3
- Quiz on the Topic 4
Demonstration / Labs relating to factors
Video: Factors Affecting Reaction Rates
Subscribe to:
Posts (Atom)


 =>
=>